Confidence in design.
Risk in control.
RD8 is a trusted partner in drug delivery device development - with a proven portfolio of products on the market and a strong record of tackling complex engineering challenges.
We deliver premium consulting in device development and R&D execution. Our work not only solves problems, it lifts your team’s capability to design, execute, and perform at a higher level.

























Focused expertise for the toughest drug delivery challenges

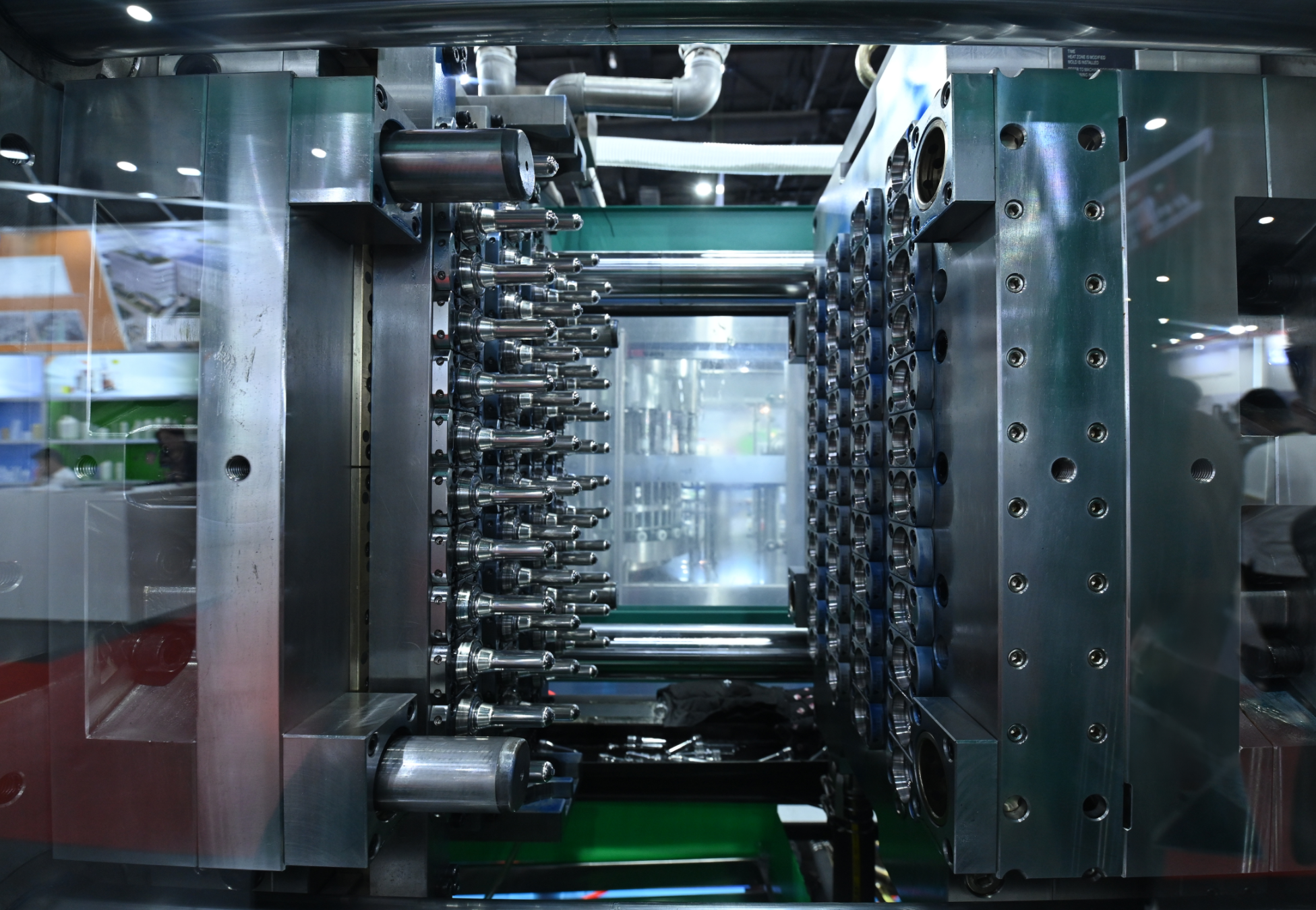
.png)
How we partner with you
Every engagement is built around your team’s needs - from focused design reviews to long-term collaboration. We bring structure, clarity, and mechanical engineering depth, so you gain both immediate results and lasting capability.
Client results
We helped a European medical device manufacturer secure a smooth production ramp-up by conducting a Robust Design assessment of a diabetes care device before release.
We worked with a US-based pharma R&D team of 40+ engineers to continuously monitor and improve injection device design.
We enabled a global device R&D and manufacturing unit to scale production sixfold — with zero re-runs on tooling and manufacturing qualification.
We guided an executive make/buy decision for injection devices through full 8-discipline assessments and KPI benchmarking.
We supported a MedTech R&D team in releasing a new apparatus four months ahead of schedule, implementing visual performance tracking to raise team execution.
We trained and coached an R&D and project management organization, embedding robust development practices while revamping core processes.


Fit first, solutions second
Our best work happens when the challenge and collaboration are right. If you want to explore whether RD8 is the right partner, let’s start there.
Talk to an expert

By submitting, you accept RD8's Privacy Policy and Terms of Service.
